Synthesis of (benzenesulfonyl)difluoromethyl thioethers from ((difluoromethyl)sulfonyl)benzene and organothiocyanates generated in situ†
Abstract
Easily available aryl and alkyl thiocyanates were converted into the corresponding (benzenesulfonyl)difluoromethyl thioethers via the direct nucleophilic substitution of ((difluoromethyl)-sulfonyl)benzene under transition metal free conditions. Combined with various thiocyanation methods, this reaction can allow late-stage (benzenesulfonyl)difluoromethylthiolation of alkyl halides and aryl diazonium salts.
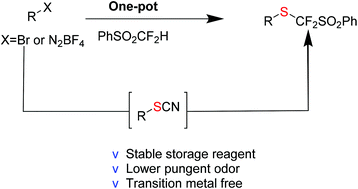
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...