Copper-catalyzed domino synthesis of ynamines†
Abstract
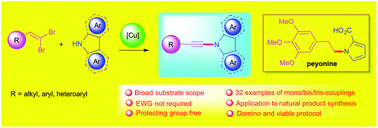
The hitherto unexplored N-alkynylation of electron-withdrawing or protecting group free N-heteroarenes such as indole, carbazole and pyrrole was developed using gem-dibromoalkenes to synthesize ynamines under ligand-free copper-catalyzed domino conditions. The development methodology of ynamines was also applied in the synthesis of enynamines, multi-coupled bis-ynamines, tris-ynamines, and the natural product peyonine, demonstrating its broad synthetic scope and applications.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...