Enantioselective vinylogous aldol reaction of acylphosphonates with 3-alkylidene oxindoles†
Abstract
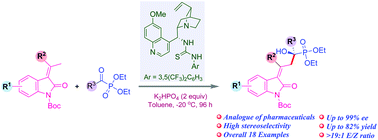
A simple strategy for yielding chiral tertiary α-hydroxy phosphonates that integrates two highly biologically relevant scaffolds namely 3-alkylidene-2-oxindoles and phosphonates has been described. The hydrogen bonding ability of the bifunctional thiourea catalyst allows simultaneous dual activation of a vinylogous oxindole nucleophile and an acylphosphonate electrophile, affording hydroxyphosphonato-3-alkylidene-2-oxindoles as aldol adducts in high yields (up to 92%) with excellent stereocontrol (up to 99% ee).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...