Spontaneous conversion of prenyl halides to acids: application in metal-free preparation of deuterated compounds under mild conditions†
Abstract
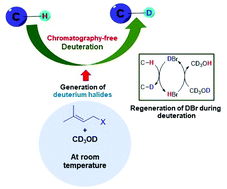
Here we reveal a simple generation of deuterium halide (DX) from common and inexpensive reagents readily available in a synthetic chemistry laboratory, i.e. prenyl-, allyl-, and propargyl halides, under mild conditions. We envisaged that in situ generation of an acid, deuterium halide, would be useful for acid-catalyzed reactions and could be employed for organocatalytic deuteration. The present work reports a metal-free method for deuterium labeling covering a broad range of substrate including phenolic compounds (i.e. flavonoids and stilbenes), indoles, pyrroles, carbonyl compounds, and steroids. This method was also applied for commonly used drugs such as loxoprofen, haloperidol, stanolone, progesterone, androstenedione, donepezil, ketorolac, adrenosterone, cortisone, pregnenolone, and dexamethasone. A gram-scale chromatography-free synthesis of some deuterated compounds is demonstrated in this work. This work provides a simple, clean and by-product-free, site-selective deuteration, and the deuterated products are obtained without chromatographic separation. When applying these initiators for other acid-catalyzed reactions, the deuterium isotope effects of DX may provide products which are different from those obtained from reactions using common acids. Although the mechanism of the spontaneous transformation of prenyl halides to acid is unclear, this overlooked chemistry may be useful for many reactions.


 Please wait while we load your content...
Please wait while we load your content...