Novel fleximer pyrazole-containing adenosine analogues: chemical, enzymatic and highly efficient biotechnological synthesis†
Abstract
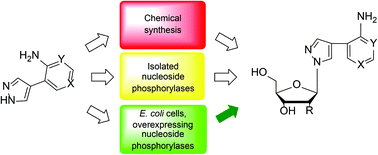
Nucleoside analogues have long served as key chemotherapeutic drugs for the treatment of viral infections and cancers. Problems associated with the development of drug resistance have led to a search for the design of nucleosides capable of bypassing point mutations in the target enzyme's binding site. As a possible answer to this, the Seley-Radtke group developed a flexible nucleoside scaffold (fleximers), where the heterocyclic purine base is split into its two components, i.e. pyrimidine and imidazole. Herein, we present a series of new pyrazole-containing flex-bases and the corresponding fleximer analogues of 8-aza-7-deaza nucleosides. Subsequent studies found that pyrazole-containing flex-bases are substrates of purine nucleoside phosphorylase (PNP). We have compared the chemical synthesis of fleximers and enzymatic approaches with both isolated enzymes and the use of E. coli cells overproducing PNP. The latter provided stereochemically pure pyrazole-containing β-D-ribo- and β-D-2′-deoxyribo-fleximers and are beneficial in terms of environmental issues, are more economical, and streamline the steps required from a chemical approach. The reaction is carried out in water, avoiding hazardous chemicals, and the products are isolated by ion-exchange chromatography using water/ethanol mixtures for elution. Moreover, the target nucleosides were obtained on a multi-milligram scale with >97–99% purity, and the reactions can be easily scaled up.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...