Two approaches for the synthesis of levo-praziquantel†
Abstract
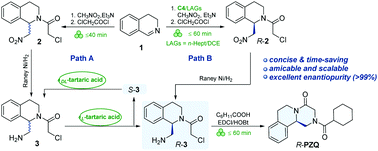
We report herein the development of two pathways for the preparation of levo-praziquantel (R-PZQ), which involves three-/four-step processes of a mechanochemical (asymmetric) aza-Henry/acylation reaction, a hydrogenation reaction, (chiral resolution) and a solvent-free acylation-ring closing reaction. The key intermediate (R)-1-aminomethyl tetrahydroisoquinoline could be obtained either by chiral resolution with a rational reuse of the S-isomer or by mechanochemical enantioselective synthesis that refrained from using a bulky toxic solvent. The efficiency and scalability of both the developed routes were demonstrated and desired target product was obtained in a satisfactory yield with excellent enantiopurity (>99%), offering practical, concise and environmentally friendly alternatives to access R-PZQ.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...