Binaphthyl–prolinol chiral ligands: design and their application in enantioselective arylation of aromatic aldehydes†
Abstract
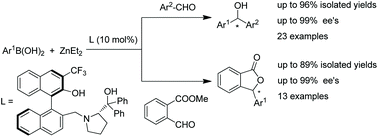
Binaphthyl–prolinol ligands were designed and applied in enantioselective arylation of aromatic aldehydes and sequential arylation–lactonization of methyl 2-formylbenzoate. Under optimized conditions, the reactions provided the desired diarylmethanols and 3-aryl phthalides in up to 96% yields with up to 99% ee and up to 89% yields with up to 99% ee, respectively. In particular, essentially optically pure 3-aryl phthalides (over 99% ee) were obtained in large quantities through recrystallization.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...