Gold-catalyzed synthesis of 1H-isochromene-4-carbaldehydes via oxidative cascade cyclization†
Abstract
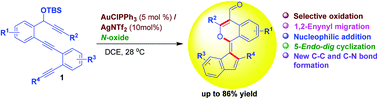
An efficient gold-catalyzed formation of indenylidene-derived 1H-isochromene-4-carbaldehydes from substituted 1,5,10-triyne-O-silanes was developed under mild reaction conditions. In this reaction, gold-catalyzed selective oxidation, 1,2-migration, nucleophilic addition and then 5-endo-dig cyclization took place regioselectively. The indenylidene-derived isochromene-4-carbaldehydes were synthesized in moderate to very good yields via the formation of new C–C and C–O bonds in one pot.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...