Regiocontrolled synthesis of 2,4,6-triarylpyridines from methyl ketones, electron-deficient acetylenes and ammonium acetate†
Abstract
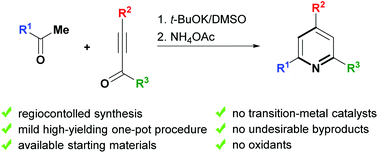
A novel one-pot two-step approach for the synthesis of 2,4,6-triarylpyridines via t-BuOK/DMSO-promoted C-vinylation of a variety of methyl ketones with electron-deficient acetylenes (alkynones) followed by a cyclization of the in situ generated unsaturated 1,5-dicarbonyl species with ammonium acetate has been developed. This approach possesses competitive advantages such as high regioselectivity, available starting materials and the absence of transition-metal catalysts, oxidants and undesirable byproducts. A wide synthetic utility of the developed approach was demonstrated by the synthesis of trisubstituted, tetrasubstituted and fused pyridines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...