Ring-fused dimethoxybenzimidazole-benzimidazolequinone (DMBBQ): tunable halogenation and quinone formation using NaX/Oxone†
Abstract
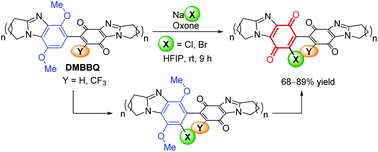
Ring-fused benzimidazolequinones are well-known anti-tumour agents, but dimeric ring-fused adducts are new. The alicyclic [1,2-a] ring-fused dimethoxybenzimidazole-benzimidazolequinone (DMBBQ) intermediate allows late-stage functionalization of bis-p-benzimidazolequinones. DMBBQs are chlorinated and brominated at the p-dimethoxybenzene site using nontoxic sodium halide and Oxone in HFIP/water. X-ray crystallography is used to rationalize site preference in terms of the discontinuity in conjugation in the DMBBQ system. Quinone formation occurs by increasing in situ halogen generation and water. Conversely, radical trifluoromethylation occurs at the quinone of the DMBBQ.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...