Synthesis of methanesulfone-containing tetrasubstituted carbon stereocenters†
Abstract
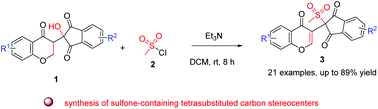
A methanesulfonylation reaction for the synthesis of sulfone-containing tetrasubstituted carbon stereocenters is described for the first time by simple treatment of indanedione–chromanone synthons with Et3N and easily accessible MsCl without any use of organometallic chemistry. This technology gave the corresponding valuable chromone-based 2-methanesulfonylated 1,3-indanediones in good yields (up to 89% yield) under mild conditions. The present work provides an attractive strategy for the construction of biologically interesting sulfone-containing tetrasubstituted carbon stereocenters, which might be valuable in medicinal chemistry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...