Synthesis of benzofused cyclobutaoxepanones via intramolecular annulation of o-cinnamyl chalcones†
Abstract
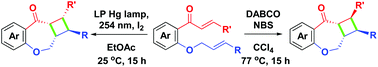
Intramolecular stereoselective annulation of o-cinnamyloxy chalcones provides two kinds of tricyclic benzofused cyclobutaoxepanones via the synthesized routes of DABCO/NBS (1,4-diazabicyclo[2.2.2]octane/N-bromosuccinimide)-mediated Baylis–Hillman type cyclization or low-pressure mercury (LP Hg) lamp-promoted photocontrolled [2 + 2] cycloaddition. Diversified reaction conditions have been investigated for one-pot facile, high-yield transformation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...