Allylation and propargylation of aldehydes mediated by in situ generated zinc from the redox couple of Al and ZnCl2 in 2N HCl†
Abstract
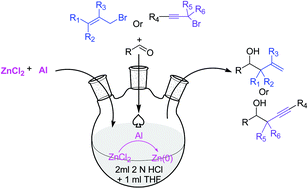
A simple one pot allylation and propargylation of aldehydes mediated by zinc(0), which is in situ generated from the redox couple of Al and ZnCl2 in 2N HCl, is demonstrated to afford the corresponding homoallyl and homopropargyl alcohols with excellent yields.


 Please wait while we load your content...
Please wait while we load your content...