Synthesis and structure of thienyl Fischer carbene complexes of PtII for application in alkyne hydrosilylation†
Abstract
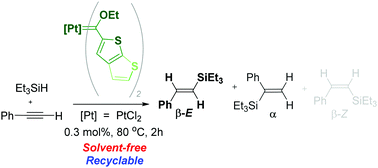
Transmetallation of group 6 thienylene Fischer carbene complexes to PtII precursors yielded new examples of neutral platinum(II) bisethoxycarbene complexes with either 2-thienyl (T) or 5-thieno[2,3-b]thienylene (TT) carbene substituents. The use of analogous aminocarbene group 6 precursors proceeded to give isomeric platinum(II) product mixtures where the resultant bisaminocarbene ligands displayed different orientations due to restricted rotation around the Pt–aminocarbene bond caused by the sterically demanding TT substituents. The well-defined PtII ethoxycarbene complexes were screened as catalyst precursors in the benchmark hydrosilylation reaction employing phenylacetylene and triethylsilane substrates. Marked selectivity for the β-E isomer (E)-triethyl(styryl)silane was observed, and the (pre)catalysts proved recyclable, active in solvent-free reactions, and displaying a high alkyne functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...