Metal-free S HN cross-coupling of pyridines with phosphine chalcogenides: polarization/deprotonation/oxidation effects of electron-deficient acetylenes†
Abstract
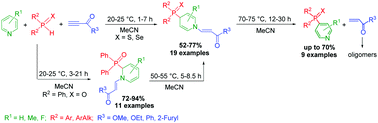
Terminal acylacetylenes, typical electron-deficient acetylenes, drive SHN cross-coupling of pyridines with secondary phosphine chalcogenides under metal-free mild conditions (20–75 °C) to afford 4-chalcogenophosphorylpyridines in up to 70% yield. The reaction proceeds via 2,4-migration of chalcogenophosphoryl groups in the intermediate 1-acylvinyl-2-phosphoryl dihydropyridines with simultaneous redox elimination of the vinyl ketone oligomers. These results are generalized in a concept of trimodal (polarization/deprotonation/oxidation) catalyst-like assistance of electron-deficient acetylenes in SHN reaction of the pyridinoid heterocycles with PH-nucleophiles, which comprises: (i) repolarization (umpolung) of the pyridine ring, (ii) deprotonation of secondary phosphine chalcogenides to generate phosphorus-centered anions and (iii) oxidation of the dihydro intermediates.


 Please wait while we load your content...
Please wait while we load your content...