From mono-rings to bridged bi-rings to caged bi-rings: a promising design strategy for all-nitrogen high-energy-density materials N10 and N12†
Abstract
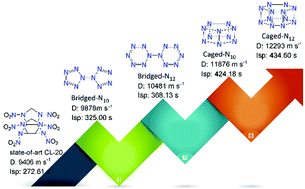
The research of all-nitrogen compounds has always been a hot topic in nitrogen chemistry and high-energy-density material communities. This research mainly focuses on acyclic and monocyclic all-nitrogen derivatives, while the bicyclic systems of all-nitrogen materials have been rarely investigated. In this study, four bicyclic all-nitrogen derivatives, viz bridged N10 and N12, and caged N10 and N12, are presented. Caged-N10 and caged-N12 exhibit much higher density (d: 1.84 and 1.89 g cm−3) and higher heats of formation (Hf: 15.19 and 16.41 kJ g−1) than bridged-N10 and bridged-N12 (d: 1.71 and 1.72 g cm−3; Hf: 7.64 and 10.24 kJ g−1), respectively. All these materials exhibit remarkable detonation performance (D: 9.87–12.29 km s−1; P: 39.41–72.26 GPa) and excellent specific impulses (Isp: 325.00–434.60 s), which is superior to the state-of-art CL-20 (D: 9.40 km s−1; P: 44.60 GPa; Isp: 272.61 s), endowing these materials with great potential as promising explosives and propellants. In addition, molecular electrostatic potentials, frontier molecular orbitals, and noncovalent interactions were studied to investigate their structure–property relationship.


 Please wait while we load your content...
Please wait while we load your content...