Theoretical study of chloride complexes with hybrid macrocycles†
Abstract
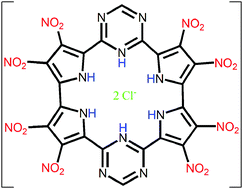
Anions show relevant roles in biological routes. The supramolecular chemistry investigates the chemical bonding between two or more molecules and/or ions. Herein, the nature of the bond between chloride anions and macrocycle receptors elaborated from (i) pyridines, (ii) pyrroles, (iii) borazines, (iv) triazines, and (v) 1,2,3-triazole rings are studied. The energy decomposition analysis (EDA) shows that the receptors that predominantly establish non-covalent interactions with the Cl− anions proportionate a preferable bond than the macrocycles that mostly form a covalent interaction with the Cl− anions. The substitution of pyridine by borazine rings in the macrocycles or the protonation of the receptors increases the interaction with the Cl− anions since there is an increase in the number of –BH or –NH groups available to establish hydrogen bonds with the Cl− anions. In addition, the pyridine → borazine substitution decreases the number of repulsive 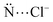 interactions. The substitution of pyrrole by 1,2,3-triazole rings does not relevantly favor the interaction with the Cl− anions. The substitution of pyridine by the triazine rings or the addition of electron-withdrawing groups (–OH, –F and –NO2) in the receptor structures increases the acidity of the cavity of the macrocycles and, therefore, favors the interaction with the Cl− anions. The addition of electron-donating groups (–NH2) to the receptor structure promotes the opposite effect. Accordingly, the present investigation brings relevant information for the design of new hybrid macrocycles with the potential for anionic recognition.
interactions. The substitution of pyrrole by 1,2,3-triazole rings does not relevantly favor the interaction with the Cl− anions. The substitution of pyridine by the triazine rings or the addition of electron-withdrawing groups (–OH, –F and –NO2) in the receptor structures increases the acidity of the cavity of the macrocycles and, therefore, favors the interaction with the Cl− anions. The addition of electron-donating groups (–NH2) to the receptor structure promotes the opposite effect. Accordingly, the present investigation brings relevant information for the design of new hybrid macrocycles with the potential for anionic recognition.


 Please wait while we load your content...
Please wait while we load your content...