Thiophenic silicon phthalocyanines: synthesis, characterization, and photophysical properties†
Abstract
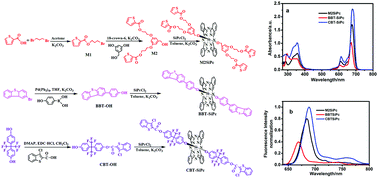
Three new thienyl/benzothienyl/dibenzothienyl groups axially substituted silicon(IV) phthalocyanines were synthesized. The structures of these thiophenic silicon(IV) phthalocyanines were characterized. The influence of different thiophenic substituents on the photophysical/photochemical properties of phthalocyanines was investigated by fluorescence and UV/Vis absorption spectroscopies. Among them, benzothienyl group substituted silicon(IV) phthalocyanine possessed the highest fluorescence intensity, lifetime, and singlet oxygen quantum yield. Benzothienyl group substituted silicon(IV) phthalocyanine could be transported into MCF-7 breast cancer cells and was not evenly distributed in the cytoplasm, and mediated photodynamic therapy to kill cancer cells.


 Please wait while we load your content...
Please wait while we load your content...