A novel human arterial wall-on-a-chip to study endothelial inflammation and vascular smooth muscle cell migration in early atherosclerosis†
Abstract
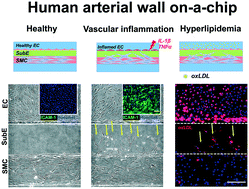
Mechanistic understanding of atherosclerosis is largely hampered by the lack of a suitable in vitro human arterial model that recapitulates the arterial wall structure, and the interplay between different cell types and the surrounding extracellular matrix (ECM). This work introduces a novel microfluidic endothelial cell (EC)–smooth muscle cell (SMC) 3D co-culture platform that replicates the structural and biological aspects of the human arterial wall for modeling early atherosclerosis. Using a modified surface tension-based ECM patterning method, we established a well-defined intima–media-like structure, and identified an ECM composition (collagen I and Matrigel mixture) that retains the SMCs in a quiescent and aligned state, characteristic of a healthy artery. Endothelial stimulation with cytokines (IL-1β and TNFα) and oxidized low-density lipoprotein (oxLDL) was performed on-chip to study various early atherogenic events including endothelial inflammation (ICAM-1 expression), EC/SMC oxLDL uptake, SMC migration, and monocyte–EC adhesion. As a proof-of-concept for drug screening applications, we demonstrated the atheroprotective effects of vitamin D (1,25(OH)2D3) and metformin in mitigating cytokine-induced monocyte–EC adhesion and SMC migration. Overall, the developed arterial wall model facilitates quantitative and multi-factorial studies of EC and SMC phenotype in an atherogenic environment, and can be readily used as a platform technology to reconstitute multi-layered ECM tissue biointerfaces.


 Please wait while we load your content...
Please wait while we load your content...