N-Phenylputrescine (NPP): a natural product inspired amine donor for biocatalysis†
Abstract
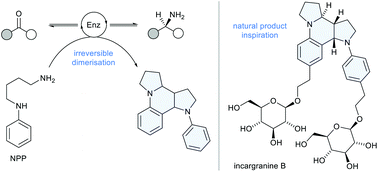
The synthesis of chiral amines in enantioenriched form is a keystone reaction in applied chemical synthesis. There is a strong push to develop greener and more sustainable alternatives to the metal-catalysed methods currently used in the pharmaceutical, agrochemical and fine chemical industries. A biocatalytic approach using transaminase (TA or ATA) enzymes to convert prochiral ketones to chiral amines with unparalleled levels of enantioselectivity is highly appealing. However, the use of TA enzymes in synthesis is severely hampered by the unfavourable thermodynamics associated with the amine donor/acceptor equilibrium. Several ‘smart’ amine donors have been developed that leverage chemical and physical driving forces to overcome this challenging equilibrium. Alongside this strategy, enzyme engineering is typically required to develop TAs compatible with these non-physiological amine donors and the unnatural reaction conditions they require. We herein disclose N-phenylputrescine (NPP) as a readily accessible amine donor, inspired by the biosynthesis of the dipyrroloquinoline alkaloids. NPP is compatible with a broad range of synthetically useful TA biocatalysts and performs across an unparalleled variety of reaction conditions (pH and temperature). Synthetic applicability has been demonstrated through the synthesis of the anti-diabetic drug sitagliptin, delivering the product in excellent enantiopurity using just two equivalents of NPP.
- This article is part of the themed collection: Biocatalysis: A cross-journal collection


 Please wait while we load your content...
Please wait while we load your content...