Cu/N-Oxyl-catalyzed aerobic oxidative esterification to oxalic acid diesters from ethylene glycol via highly selective intermolecular alcohol oxidation†
Abstract
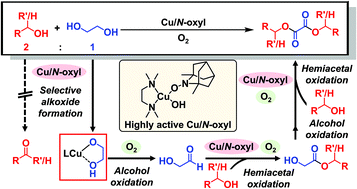
One of the ideal green esterification reactions is aerobic oxidative esterification using only a stoichiometric amount of different alcohols via intermolecular selective alcohol oxidation followed by hemiacetal formation by the addition of the other alcohol and hemiacetal oxidation to esters. However, oxalic acid diester synthesis via oxidative esterification has not been reported to date, possibly owing to the difficulty of selectivity control of intermolecular alcohol oxidation and the chelating effects of ethylene glycol-derived alcohols/hemiacetals on inhibiting oxidation catalysts. Herein, using a CuCl/tetramethylethylenediamine/1,5-dimethyl-9-azanoradamantane N-oxyl catalyst, we describe a highly efficient aerobic oxidative esterification reaction of ethylene glycol to various oxalic acid diesters via selective oxidation of ethylene glycol-derived alcohols/hemiacetals even in the presence of other aliphatic primary alcohols. Notably, the green reaction works well using an ideal stoichiometric ratio of ethylene glycol and primary/secondary alcohols. Thorough experimental investigation and theoretical calculations revealed that highly selective oxidative esterification is enabled by the preferential bidentate coordination of ethylene glycol-derived alcohols/hemiacetals to the Cu(II) species, followed by efficient two-electron/one-proton transfer.


 Please wait while we load your content...
Please wait while we load your content...