High-efficiency electrochemical nitrite reduction to ammonium using a Cu3P nanowire array under ambient conditions†
Abstract
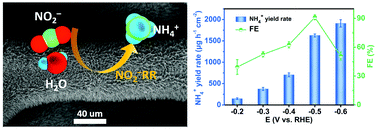
Electrochemical reduction of nitrite addresses the need for nitrite contaminant removal and provides an on-site, cost-effective, and sustainable route for rapidly generating ammonia/ammonium. Its practical implementation however requires advanced electrocatalysts with enhanced ammonium yield rates and faradaic efficiencies. Our study here introduces a Cu3P nanowire array supported on copper foam (Cu3P NA/CF) for the first time as an efficient electrocatalyst for nitrite-to-ammonium conversion in neutral media. In 0.1 M phosphate buffered saline containing 0.1 M NaNO2, the freestanding Cu3P NA/CF electrode displays a large ammonium yield rate of 1626.6 ± 36.1 μg h−1 cm−2 and a high FE of 91.2 ± 2.5% at −0.5 V vs. a reversible hydrogen electrode, with good stability. The catalytic mechanism is revealed by density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...