A catalytic approach via retro-aldol condensation of glucose to furanic compounds†
Abstract
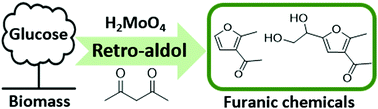
The synthesis of new types of furan-based compounds other than 5-hydroxymethylfurfural from glucose is a very attractive yet underexploited strategy. We report here a catalytic conversion of glucose with acetylacetone (acac) to furan-centered chemicals, 2-methyl-3-acetylfuran (MAF) and 1-(5-(1,2-dihydroxyethyl)-2-methylfuran-3-yl)ethan-1-one (DMAF), which are potential building blocks for the synthesis of fine chemicals. The experimentally supported reaction mechanism is cascade-type, including glycolaldehyde (GA) formation by H2MoO4-catalysed retro-aldol condensation (C2 + C4) of glucose and immediate capture of transient C2 and C4 intermediates by acac to yield MAF and DMAF. To the best of our knowledge, this is the first report on the straightforward synthesis of MAF and DMAF from glucose, providing a new but generic synthesis strategy for GA-based C2 and erythrose-based C4 chemistry in biorefining.


 Please wait while we load your content...
Please wait while we load your content...