Metal-free regioselective bromination of imidazo-heteroarenes: the dual role of an organic bromide salt in electrocatalysis†
Abstract
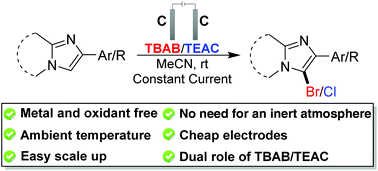
This paper represents an electrochemical transformation by demonstrating the dual role of an organic bromide salt, i.e., tetra-n-butylammonium bromide (TBAB), as a brominating agent and as an electrolyte for the regioselective bromination of several imidazo heteroaromatic motifs. Instead of using a transition metal/external oxidant, this methodology utilized electron holes and electrons by means of anodic oxidation and cathodic reduction to form the desired products in good to excellent yields at ambient temperature. The method is simple, environment friendly and compatible with various functional groups. The significance of this sustainable greener bromination technique relies on the fact that the readily available cost-effective electrodes (C(+)/C(−)) can be reused up to forty times without loss of any electrochemical activities. The electro-oxidative method could efficiently be scaled up and can be extended to chlorination as well. Moreover, this electro-synthetic strategy was extrapolated to the domino organo-electrochemical bromination technique for the synthesis of a brominated imidazo heteroaromatic moiety directly starting from substituted 2-bromoacetophenone and 2-aminopyridine by using catalytic amounts of electrolyte.


 Please wait while we load your content...
Please wait while we load your content...