The polyhedral nature of selenium-catalysed reactions: Se(iv) species instead of Se(vi) species make the difference in the on water selenium-mediated oxidation of arylamines†
Abstract
Selenium-catalysed oxidations are highly sought after in organic synthesis and biology. Herein, we report our studies on the on water selenium mediated oxidation of anilines. In the presence of diphenyl diselenide or benzeneseleninic acid, anilines react with hydrogen peroxide, providing direct and selective access to nitroarenes. On the other hand, the use of selenium dioxide or sodium selenite leads to azoxyarenes. Careful mechanistic analysis and 77Se NMR studies revealed that only Se(IV) species, such as benzeneperoxyseleninic acid, are the active oxidants involved in the catalytic cycle operating in water and leading to nitroarenes. While other selenium-catalysed oxidations occurring in organic solvents have been recently demonstrated to proceed through Se(VI) key intermediates, the on water oxidation of anilines to nitroarenes does not. These findings shed new light on the multifaceted nature of organoselenium-catalysed transformations and open new directions to exploit selenium-based catalysis.
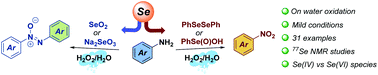


 Please wait while we load your content...
Please wait while we load your content...