An oxidant- and catalyst-free electrooxidative cross-coupling approach to 3-tetrahydroisoquinoline substituted coumarins†
Abstract
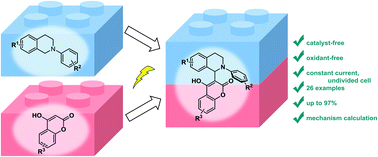
A direct electrooxidative cross-dehydrogenative-coupling (CDC) reaction between N-aryl-tetrahydroisoquinolines and 4-hydroxycoumarins has been realized. This protocol provides a green, mild and fast method to construct 3-tetrahydroisoquinoline substituted coumarins in the absence of any catalysts and exogenous oxidants. A variety of N-aryl-tetrahydroisoquinolines and 4-hydroxycoumarins are compatible with this transformation to give the corresponding products in moderate to excellent yields. Moreover, the results of control experiments, cyclic voltammetry experiments, and density functional theory (DFT) calculations indicated that the electrooxidative CDC reaction might involve both radical addition and nucleophilic addition processes.


 Please wait while we load your content...
Please wait while we load your content...