Lead minerals found in drinking water distribution systems increase chlorine dioxide decay to a single inorganic product†
Abstract
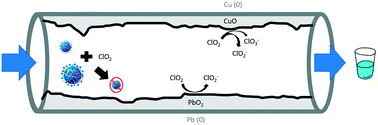
Chlorine dioxide (ClO2), an alternative disinfectant to free chlorine, has been used to reduce the formation of organic disinfection byproducts during water treatment. ClO2 is highly reactive, and its decay is enhanced in the presence of lead oxide (PbO2), lead carbonate (PbCO3), and cupric oxide (CuO), minerals commonly found in the corrosion scale on distribution system pipes. Reaction of these corrosion minerals with chlorine dioxide and the byproducts created during said reactions were investigated. In addition to corrosion mineral, initial concentration of ClO2 and pH were both varied. Reaction took place in a batch reactor, ClO2 was measured via ultraviolet-visible spectrophotometry, and byproducts were quantified using ion chromatography. Only chlorite is produced from ClO2 decay when lead minerals are present, whereas both chlorite and chlorate are produced by disproportionation when CuO is present. ClO2 decay occurs at a significantly higher rate on PbO2 compared to CuO. The reaction rate for ClO2 decay, in the presence of PbO2, peaks at pH 8.3, which corresponds to the point of zero charge of PbO2. This indicates that surface charge may play a significant role in lead mineral-assisted decay reactions with disinfectants, including ClO2. The reaction rate dependence for ClO2 decay on PbO2 is well described by a pseudo-second-order adsorption kinetic model. These findings highlight the need for chlorite, but not chlorate, byproduct management when ClO2 is used as a disinfectant in water distribution systems with lead-based pipes. Robust strategies for management of a chlorine dioxide residual must be implemented in both copper and lead-based service lines to ensure a disinfectant persists and protects against microbial contamination in premise plumbing.


 Please wait while we load your content...
Please wait while we load your content...