Defining silica–water interfacial chemistry under nanoconfinement using lanthanides†
Abstract
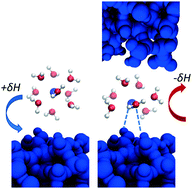
A quarter of Earth's land surface is covered by porous sedimentary silicate rocks, so silica–water interfaces are critical to the fate and transport of chemical species on a global-scale. However, while the physiochemical properties of unconfined silica–water interfaces are understood reasonably well, these properties have proven to be unpredictable when the interface is confined in nanometer-scale pores within sedimentary rocks. For example, the existing theories struggle to quantitatively predict how the energetics of adsorption reactions and the coordination environment of adsorbed species shift due to nanoconfinement of an interface. Here, we utilized gradual and known variations in the properties of trivalent lanthanide ions to decipher the chemical interactions that cause the nanoconfinement effects on chemistry at the silica–water interfaces. We discovered that the lanthanide's free energy of hydration (ΔGhydr) is a descriptor that can be used to predict the extent to which nanoconfinement will change the thermodynamics and products of interfacial reactions. We show that nanoconfinement promotes inner-sphere complexation between lanthanides and silica surface, as well as the formation of polymeric surface species. In nanoconfined domains lanthanide's ΔGhydr becomes less negative, reducing the energy required to dehydrate the ion during the formation of an inner-sphere surface complex. These nanoconfinement effects on chemistry become more pronounced for ions with lower hydration free energies.


 Please wait while we load your content...
Please wait while we load your content...