Reactions of five-membered zirconacycloalkynes and zirconacycloallenes with Cp2Zr(H)Cl; formal hydrogenation by metal hydrides†‡
Abstract
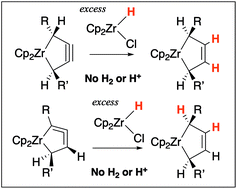
Reactions of five-membered zirconacycloalkynes and zirconacyloallenes (1-zirconacyclopent-3-ynes and 1-zirconacyclopenta-2,3-dienes) with an excess of Cp2Zr(H)Cl, known as the Schwartz reagent, were studied. Both reactions gave five-membered zirconacycloalkenes, 1-zirconacyclopent-3-enes without subsequent work-up such as protonolysis and hydrogenolysis. The product was identical to the zirconocene-diene complex that was prepared from Cp2Zr(n-Bu)2 (Negishi reagent) and the corresponding 1,4-disubstituted 1,3-dienes. These results indicate that formal hydrogenation by metal hydride took place. The use of diisobutylaluminum hydride or 9-borabicyclo[3.3.1]nonane also gave the same product, albeit in lower yields. The reactions starting from deuterated compounds suggested that double hydrozirconation followed by elimination of a dinuclear zirconium complex resulted in the hydrogenated products.


 Please wait while we load your content...
Please wait while we load your content...