Formation of a hydride containing amido-zincate using pinacolborane†
Abstract
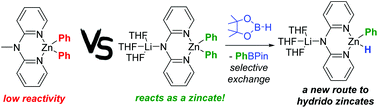
Amido-zincates containing hydrides are underexplored yet potentially useful complexes. Attempts to access this type of zincate through combining amido-organo zincates and pinacolborane (HBPin) via Zn–C/H–BPin exchange led instead to preferential formation of amide–BPin and/or [amide–BPin(Y)]− (Y = Ph, amide, H), when the amide is hexamethyldisilazide or 2,2,6,6-tetramethylpiperidide and the hydrocarbyl group was phenyl or ethyl. In contrast, the use of a dipyridylamide (dpa) based arylzinc complex led to Zn–C/H–BPin metathesis being the major outcome. Independent synthesis and full characterisation of two LnLi[(dpa)ZnPh2] (L = THF, n = 3; L = PMDETA, n = 1) complexes, 1 and 3, respectively, enabled reactivity studies that demonstrated that these species display zincate type reactivity (by comparison to the lower reactivity of the neutral complex (Me-dpa)ZnPh2, 4, Me-dpa = 2,2′-dipyridyl-N-methylamine). This included 1 performing the rapid deprotonation of 4-ethynyltoluene and also phenyl transfer to α,α,α-trifluoroacetophenone in contrast to neutral complex 4. Complex 1 reacted with one equivalent of HBPin to give predominantly PhBPin (ca. 90%) and a lithium amidophenylzincate containing a hydride unit, complex 7-A, as the major zinc containing product. Complex 7-A transfers hydride to an electrophile preferentially over phenyl, indicating it reacts as a hydridozincate. Attempts to react 1 with >1 equivalent of HBPin or with catecholborane led to more complex outcomes, which included significant borane and dpaZn substituent scrambling, two examples of which were crystallographically characterised. While this work provides proof of principle for Zn–C/H–BPin exchange as a route to form an amido-zincate containing a hydride, amido-organozincates that undergo more selective Zn–C/H–BPin exchange still are required.


 Please wait while we load your content...
Please wait while we load your content...