Magneli-type tungsten oxide nanorods as catalysts for the selective oxidation of organic sulfides†
Abstract
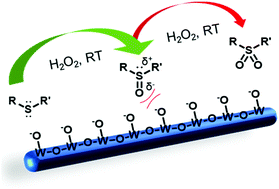
Selective oxidation of thioethers is an important reaction to obtain sulfoxides as synthetic intermediates for applications in the chemical industry, medicinal chemistry and biology or the destruction of warfare agents. The reduced Magneli-type tungsten oxide WO3−x possesses a unique oxidase-like activity which facilitates the oxidation of thioethers to the corresponding sulfoxides. More than 90% of the model system methylphenylsulfide could be converted to the sulfoxide with a selectivity of 98% at room temperature within 30 minutes, whereas oxidation to the corresponding sulfone was on a time scale of days. The concentration of the catalyst had a significant impact on the reaction rate. Reasonable catalytic effects were also observed for the selective oxidation of various organic sulfides with different substituents. The WO3−x nanocatalysts could be recycled at least 5 times without decrease in activity. We propose a metal oxide-catalyzed route based on the clean oxidant hydrogen peroxide. Compared to other molecular or enzyme catalysts the WO3−x system is a more robust redox-nanocatalyst, which is not susceptible to decomposition or denaturation under standard conditions. The unique oxidase-like activity of WO3−x can be used for a wide range of applications in synthetic, environmental or medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...