Ensemble effects on allylic oxidation within explicit solvation environments†
Abstract
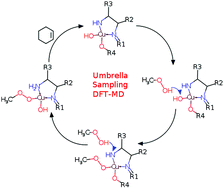
Umbrella-sampling density functional theory molecular dynamics (DFT-MD) has been employed to study the full catalytic cycle of the allylic oxidation of cyclohexene using a Cu(II) 7-amino-6-((2-hydroxybenzylidene)amino)quinoxalin-2-ol complex in acetonitrile to create cyclohexenone and H2O as products. After the initial H-atom abstraction step, two different reaction pathways have been identified that are distinguished by the participation of alkyl hydroperoxide (referred to as the “open” cycle) versus the methanol side-product (referred to as the “closed” cycle) within the catalyst recovery process. Importantly, both pathways involve dehydrogenation and re-hydrogenation of the –NH2 group bound to the Cu-site – a feature that is revealed from the ensemble sampling of configurations of the reactive species that are stabilized within the explicit solvent environment of the simulation. Estimation of the energy span from the experimental turnover frequency yields an approximate value of 22.7 kcal mol−1 at 350 K. Whereas the closed cycle value is predicted to be 26.2 kcal mol−1, the open cycle value at 16.5 kcal mol−1. Both pathways are further consistent with the equilibrium between Cu(II) and Cu(III) that has previously been observed. In comparison to prior static DFT calculations, the ensemble of both solute and solvent configurations has helped to reveal a breadth of processes that underpin the full catalytic cycle yielding a more comprehensive understanding of the importance of radical reactions and catalysis recovery.


 Please wait while we load your content...
Please wait while we load your content...