Abstract
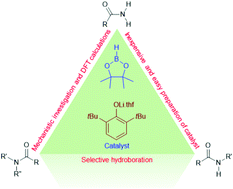
A selective and efficient route for the deoxygenative reduction of primary to tertiary amides to corresponding amines has been achieved with pinacolborane (HBpin) using simple and readily accessible 2,6-di-tert-butyl phenolate lithium·THF (1a) as a catalyst. Both experimental and DFT studies provide mechanistic insight.


 Please wait while we load your content...
Please wait while we load your content...