Electrochemical water oxidation by a copper complex with an N4-donor ligand under neutral conditions†
Abstract
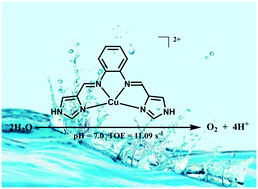
Herein, electrochemical water oxidation catalyzed by a copper(II) complex [CuII(H2L)](NO3)2 with the redox-active salophen-like N4-donor ligand N,N′-bis-(1H-imidazol-4-yl)methylidene-o-phenylenediamine is demonstrated. Oxygen evolution with a high turnover frequency of 11.09 s−1 and a low onset overpotential of only 580 mV is achieved in neutral phosphate buffer solution, and [CuII(H2L)]2+ is confirmed as an efficient molecular water oxidation catalyst with long-term stability. The results of electrochemical tests provide evidence that the Cu center undergoes a water nucleophilic attack process and participates in the catalytic cycle. The subsequent one-step proton-coupled electron transfer (PCET) process of the Cu center and the two-step PCET process of the ligand are both critical for efficient water oxidation. This work indicates that the ligand assisted catalytic cycle is a favorable method for the accumulation of intermediate species that account for electrochemical water oxidation.


 Please wait while we load your content...
Please wait while we load your content...