Kinetic and structural understanding of bulk and supported vanadium-based catalysts for furfural oxidation to maleic anhydride†
Abstract
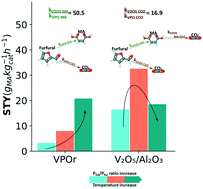
The kinetics of gas-phase furfural partial oxidation to maleic anhydride (MA) was studied over bulk vanadium–phosphorus-based catalysts obtained by aqueous (VPAq) and organic (VPOr) methods and compared to a supported V2O5/Al2O3 catalyst. The solids were characterized by N2 adsorption–desorption, XRD and UV-vis DRS. Results showed a higher specific surface area on VPOr compared with VPAq materials, with a well-defined (VO)2P2O7 crystalline structure. UV-vis analysis showed mainly V(V) on VPAq and an intermediate state between V(IV) and V(V) on VPOr. A detailed kinetic study demonstrated that furfural can be oxidized to MA or COx through parallel paths. At high oxygen partial pressures MA oxidation is inhibited on VPO catalysts but favored on V2O5/Al2O3. A Langmuir–Hinshelwood kinetic model with negligible site occupancy fits the experimental data with a 16% mean error. It also shows a higher apparent activation energy for furfural partial oxidation than for complete oxidation, highlighting the favored selectivity to maleic anhydride at higher temperatures on VPO catalysts.


 Please wait while we load your content...
Please wait while we load your content...