Immobilization of a Pd(ii)-containing N-heterocyclic carbene ligand on porous organic polymers: efficient and recyclable catalysts for Suzuki–Miyaura reactions†
Abstract
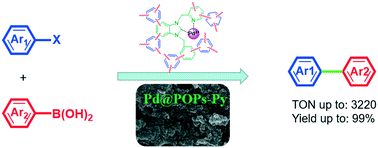
Palladium coordinated with N-heterocyclic carbene-functionalized porous organic polymers (Pd@POPs) was successfully prepared via Scholl coupling reaction and a successive immobilization method. The protocol features simple reaction conditions, easy separation, high yield and low cost. The structure and composition of Pd@POPs were characterized by N2 sorption, TGA, FT-IR, SEM, EDX, TEM, XPS and ICP. Then the obtained heterogeneous catalysts were applied in Suzuki–Miyaura coupling reaction to evaluate their catalytic performance. The Pd@POPs displayed high catalytic activity for the Suzuki–Miyaura coupling reaction in an EtOH/H2O solvent. A 0.03 mol% Pd loading was sufficient for the reaction with a high turnover number (TON) of 3220. Moreover, the catalyst was easily recovered and reused for at least six consecutive cycles without obvious loss of its initial activity.


 Please wait while we load your content...
Please wait while we load your content...