The catalytic decomposition of nitrous oxide and the NO + CO reaction over Ni/Cu dilute and single atom alloy surfaces: first-principles microkinetic modelling†
Abstract
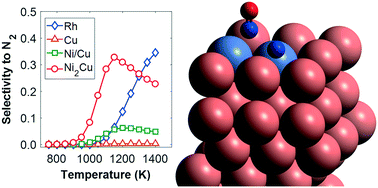
The development of platinum group metal-free (PGM-free) catalysts, which can efficiently reduce pollution-causing emissions, is an important task for overcoming major environmental challenges. In particular, nitrogen oxides (NOx) are major contributors to air pollution, being one of the culprits for smog and ozone depletion. In this work, we employ density functional theory (DFT) and microkinetic modelling to investigate the decomposition of N2O and the NO + CO reaction over two PGM-free Ni/Cu dilute alloys. On the first surface, Ni atoms are isolated on the host Cu(111), thereby forming a single atom alloy surface (i.e. Ni/Cu(111) SAA), while on the second, the same atoms are organised as Ni–Ni dimers (i.e. Ni2Cu(111)). The same reactions are also simulated on pure Cu(111) (i.e. the host surface), and on Rh(111), which is used for benchmarking as Rh is a well-established PGM in emissions control catalysis. Our results suggest that the addition of trace amounts of Ni on Cu(111) may bring about significant improvement to the catalytic performance with regard to the catalytic decomposition of N2O. Additionally, we determine that Ni2Cu(111) shows equivalent, or under some circumstances even better, performance as compared to Rh(111) for the NO + CO reaction. This work contributes to the long-standing efforts toward the design of efficient PGM-free catalytic materials for the reduction of noxious gases.


 Please wait while we load your content...
Please wait while we load your content...