Electrocatalytic dimeric inactivation mechanism by a porphyrinic molecular-type catalyst: integration in a glucose/O2 fuel cell†
Abstract
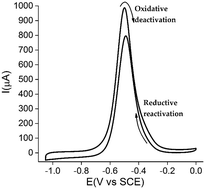
We report a chemical inactivation/redox reactivation process (IAP) based on the surface-confined rhodium–porphyrinic catalyst on a multi-walled carbon nanotube surface which presents an excellent and stable electron transfer. We used a chronoamperometric method with mathematical models and digital simulation to investigate the IAP at the catalytic metallic site. We present a mechanistic analysis of the non-catalytic and catalytic responses exhibited by this complex enabling a deep understanding of the thermodynamic and kinetic parameters that govern the IAP. These studies support a mechanism for glucose oxidation that proceeds through a complex EC′CECE scheme with catalytic steps similar to the ones reported for hydrogenases. The overall mechanism was detailed based on both electrochemical experiments and experimentally validated models. The high activity of this catalyst allows us to integrate this molecular nanomaterial in a fully molecular fuel cell together with phthalocyanine cobalt at the cathode. The resulting fuel cell reaches 0.3 mW cm−2 with a possible regeneration of initial performance.


 Please wait while we load your content...
Please wait while we load your content...