Mechanistic understanding enables chemoselective sp3 over sp2 C–H activation in Pd-catalyzed carbonylative cyclization of amino acids†
Abstract
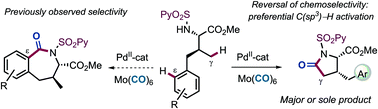
Mechanistic insights into the factors that control chemoselectivity in competing C(sp2)–H and C(sp3)–H activation pathways in the palladium-catalyzed carbonylative cyclization of γ-arylated valine type derivatives, gained by experimental observations and DFT studies, have been leveraged to reverse the remarkable selectivity of Pd for arene C(sp2)–H activation over C(sp3)–H cleavage. These studies suggest that ε-C(sp2)–H bond cleavage is significantly faster and more reversible than the γ-C(sp3)–H bond activation, whereas subsequent AcOH/CO exchange and CO insertion from the C(sp3)-palladacycle lead to more stable intermediates from which the reaction is irreversible. Control of chemoselectivity has been achieved by playing on the reaction conditions to favour thermodynamic over kinetic control. Addressing this fundamental limitation of C–H functionalization under Pd-catalysis has enabled the access to different heterocyclic frameworks (i.e., γ-lactams instead of benzazepinone skeletons) from the same starting substrate.


 Please wait while we load your content...
Please wait while we load your content...