Electropolymerized metal-protoporphyrin electrodes for selective electrochemical reduction of CO2†
Abstract
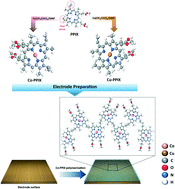
Developing catalysts that exhibit high efficiencies for the electrochemical CO2 reduction reaction (CO2RR) in aqueous media is vital both for a healthier environment and practical implementation to produce value-added fuels from energy waste. In this work, a facile electrochemical polymerization method is demonstrated to assemble porphyrin-based organometallic catalysts composed of Co(II)- or Cu(II)-protoporphyrin IX (M-PPIX) that can be employed for efficient electrocatalytic CO2RR to yield different products. The electropolymerized catalysts were first investigated in an aprotic solvent using a glassy carbon (GC) electrode to reveal the electrochemical behavior in CO2RR. We further demonstrated the efficacy of the electropolymerization method on a gas diffusion electrode (GDE) using a flow-cell configuration with an aqueous electrolyte. The oxidative saturation of the vinyl groups in the PPIX monomers was found to be responsible for linking the polymeric blocks of the catalyst films. The electropolymerized catalysts exhibited superior performance toward CO2RR over its monomeric forms. Co-PPIX was found to selectively produce CO, whereas switching the core element to Cu produced mixed gases of CO, CH4, and C2H4. The monomeric forms of the Co-PPIX catalyst demonstrated a maximum faradaic efficiency of 61% at −0.82 VRHE with a turnover frequency of 0.37 s−1 for CO production. In contrast, the catalytic performance of the electropolymerized Co-PPIX exhibited a maximum faradaic efficiency of 94% at −1.32 VRHE for CO production, with a current density of about 33 mA cm−2 and a turnover frequency of ∼1.0 s−1.


 Please wait while we load your content...
Please wait while we load your content...