Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel
Abstract
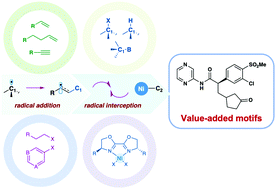
The catalytic dicarbofunctionalization of unsaturated π bonds represents a powerful platform for the rapid construction of complex motifs. Despite remarkable progress, novel and efficient methods for achieving such transformations under milder conditions with chemo-, regio-, and stereoselectivity still remain a significant challenge; thus, their development is highly desirable. Recently, the merging of nickel catalysis with radical chemistry offers a new and benign platform for the catalytic dicarbofunctionalization of unsaturated π bonds with unprecedented reactivity and selectivity. In this review, we summarize the recent advances in this area by underpinning the catalytic domino transformations involving radical capture by nickel to provide a clear overview of reaction designs and mechanistic scenarios.
- This article is part of the themed collection: Radical Chemistry


 Please wait while we load your content...
Please wait while we load your content...