Cysteinyl radicals in chemical synthesis and in nature
Abstract
Nature harnesses the unique properties of cysteinyl radical intermediates for a diverse range of essential biological transformations including DNA biosynthesis and repair, metabolism, and biological photochemistry. In parallel, the synthetic accessibility and redox chemistry of cysteinyl radicals renders them versatile reactive intermediates for use in a vast array of synthetic applications such as lipidation, glycosylation and fluorescent labelling of proteins, peptide macrocyclization and stapling, desulfurisation of peptides and proteins, and development of novel therapeutics. This review provides the reader with an overview of the role of cysteinyl radical intermediates in both chemical synthesis and biological systems, with a critical focus on mechanistic details. Direct insights from biological systems, where applied to chemical synthesis, are highlighted and potential avenues from nature which are yet to be explored synthetically are presented.
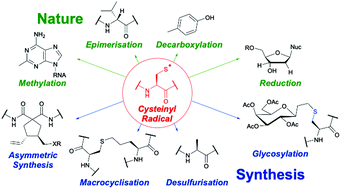
- This article is part of the themed collection: Radical Chemistry


 Please wait while we load your content...
Please wait while we load your content...