Protein confinement fine-tunes aggregation-induced emission in human serum albumin†
Abstract
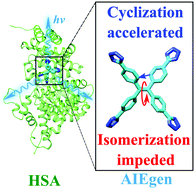
Luminogens exhibiting aggregation-induced-emission characteristics (AIEgens) have been designed as sensitive biosensors thanks to their “turn-on” fluorescence upon target binding. However, their AIE mechanism in biomolecules remains elusive except for the qualitative picture of restricted intramolecular motions. In this work, we employed ab initio simulations to investigate the AIE mechanism of two tetraphenylethylene derivatives recently developed for sensitive detection of human serum albumin (HSA) in biological fluids. For the first time, we quantified the ab initio free energy surfaces and kinetics of AIEgens to access the conical intersections on the excited state in the protein and aqueous solution, using a novel first-principles electronic structure method that incorporates both static and dynamic electron correlations. Our simulations accurately reproduce the experimental spectra and high-level correlated electronic structure calculations. We found that in HSA the internal conversion through the cyclization reaction is preferred over the isomerization around the central ethylenic double bond, whereas in the aqueous solution the reverse is true. Accordingly, the protein environment is able to moderately speed up certain non-radiative decay pathways, a new finding that is beyond the prediction of the existing model of restricted access to a conical intersection (RACI). As such, our findings highlight the complicated effects of the protein confinement on the competing non-radiative decay channels, which has been largely ignored so far, and extend the existing theories of AIE to biological systems. The new insights and the multiscale computational methods used in this work will aid the design of sensitive AIEgens for bioimaging and disease diagnosis.


 Please wait while we load your content...
Please wait while we load your content...