Theoretical insights into volatile iodine adsorption onto COF-DL229†
Abstract
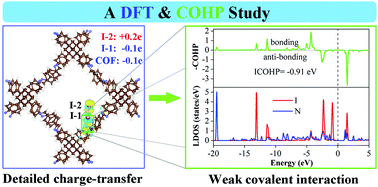
COF-DL229 is one of the promising sorbents for the capture of volatile radioiodine due to its large adsorption capacity. However, the interaction mechanism between them remains unclear. In the present work, the adsorption of volatile iodine onto COF-DL229 was systematically investigated using periodic density functional theory and crystal orbital Hamilton population calculations. The “soft” characters of COF-DL229 have been theoretically demonstrated. Furthermore, the adsorption energies are extremely large (−8.38 to −9.26 eV), which mainly originate from the framework deformation energies, accounting for 90% at least. The I2 interacts with the skeleton mainly through the N atoms of the imine linkers or the C atoms of the phenyl rings. And, the I–N bond is the strongest bond among all the potential secondary bonds formed between the skeleton and I2. The electrons could be transferred from the skeletons to the iodine atoms and from the near iodine atom to the far one. It is also found that the energy gap becomes narrow after iodine adsorption and the skeletons mainly interact with the bonding orbital σp of I2. The present work could provide reasonable theoretical explanations to the corresponding experimental investigations and contribute to the design and screening of better sorbents for the capture of volatile radioiodine.


 Please wait while we load your content...
Please wait while we load your content...