Quantum chemical assessment of the molecular area corresponding to the onset of the LE–LC phase transition for amphiphilic 2D monolayers at the air/water interface
Abstract
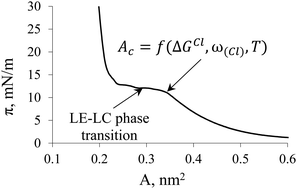
An approach for the assessment of the area per surfactant molecule in a monolayer at the onset of the LE–LC phase transition (Ac) is proposed based on the quantum chemical approach and a thermodynamic model for amphiphilic monolayers, which takes into account the nonideality of the mixing entropy. The values of the Gibbs’ clusterization energy for small surfactant associates, as well as the geometric parameters of the monolayer unit cells, were used, previously calculated using the semiempirical PM3 method for eight classes of amphiphilic compounds: saturated and ethoxylated alcohols, saturated and unsaturated cis-carboxylic acids, α-hydroxylic and α-aminoacids, N-acyl-substituted alanine and dialkyl-substituted melamine. The obtained Ac values are in satisfactory agreement with the available experimental data. This allows using the proposed approach for prognostic purposes in the cases where there are no corresponding π–A isotherms for necessary surfactants, but there are calculated thermodynamic and structural parameters of its clusterization.


 Please wait while we load your content...
Please wait while we load your content...