Time-resolved observations of vibrationally excited NO X 2Π (v′) formed from collisional quenching of NO A 2Σ+ (v = 0) by NO X 2Π: evidence for the participation of the NO a 4Π state
Abstract
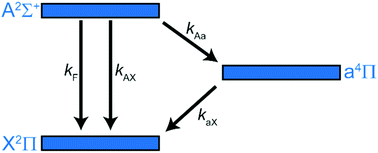
Time-resolved observations have been made of the formation of vibrationally excited NO X 2Π (v′) following collisional quenching of NO A 2Σ+ (v = 0) by NO X 2Π (v = 0). Two time scales are observed, namely a fast production rate consistent with direct formation from the quenching of the electronically excited NO A state, together with a slow component, the magnitude and rate of formation of which depend upon NO pressure. A reservoir state formed by quenching of NO A 2Σ+ (v = 0) is invoked to explain the observations, and the available evidence points to this state being the first electronically excited state of NO, a 4Π. The rate constant for quenching of the a 4Π state to levels v′ = 11–16 by NO is measured as (8.80 ± 1.1) × 10−11 cm3 molecule−1 s−1 at 298 K where the error quoted is two standard deviations, and from measurements of the increased formation of high vibrational levels of NO(X) by the slow process we estimate a lower limit for the fraction of self-quenching collisions of NO A 2Σ+ (v = 0) which lead to NO a 4Π as 19%.


 Please wait while we load your content...
Please wait while we load your content...