Fragmentation and rearrangement of Breslow intermediates: branches to both radical and ionic pathways†
Abstract
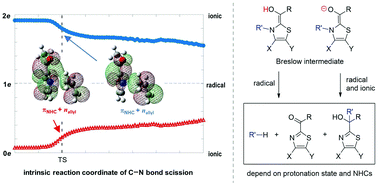
Breslow intermediates are the key species in N-heterocyclic carbene-catalyzed reactions to promote the C–C bond formation. As the fragmentation and rearrangement of Breslow intermediates terminate the catalytic cycle of N-heterocyclic carbene, two mechanisms under debate have been proposed in terms of the radical channel and the ionic route. Theoretical calculations demonstrate herein that ionic and radical characteristics can coexist, depending on the protonation state of the hydroxyl group in Breslow intermediates: radicals are merely generated in the enol system, while both ionic and radical species exist in the enolate system with a lower barrier. Complete pathways for thiamin analogue and N-allyl benzothiazole Breslow intermediates are exclusively constructed considering experimental conditions. The growing population of the enolate under higher pH values rationalizes the increased rate of the fragmentation of thiamin. The fragmentation products of thiamin, namely pyrimidine and ketone, are the thermodynamic products, while the tertiary alcohol is both the kinetic and thermodynamic product for N-allyl benzothiazole Breslow intermediate via a Claisen-like rearrangement. Other NHCs used to synthesize tertiary alcohols could form the enolate due to the base, followed by the production of stable radicals and recombination to form tertiary alcohols. It is concluded that specific protonation states and chemical structures of NHCs account for the distinct mechanisms.


 Please wait while we load your content...
Please wait while we load your content...