A high throughput dynamic method for characterizing thermodynamic properties of catalyzed magnesium hydrides by thermogravimetric analysis
Abstract
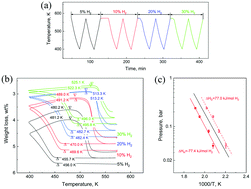
The use of the conventional pressure–composition–temperature (PCT) method to determine the thermodynamics of metal hydrides is a time-consuming process. This work presents an efficient method based on thermogravimetric analysis (TGA), to characterize the thermodynamic parameters. Through cycling catalyzed magnesium hydride in a TGA apparatus under a flowing gas with a constant hydrogen partial pressure, TGA curves can be used to determine absorption/desorption equilibrium temperatures. Based on the van’t Hoff analysis, the reaction enthalpies and entropies can be derived from the equilibrium temperatures as a function of hydrogen pressure. Using the results of this work we calculated the hydrogenation and dehydrogenation enthalpies, which are 79.8 kJ per mol per H2 and 76.5 kJ per mol per H2, respectively. These values are in good agreement with those reported values using the PCT method. These results demonstrate that the TGA can be an efficient and effective method for measuring thermodynamic parameters of metal hydrides.

 Please wait while we load your content...
Please wait while we load your content...