Modulation effects of S vacancy and Mo edge on the adsorption and dissociation behaviors of toxic gas (H2S, SO2) molecules on the MoS2 monolayer†
Abstract
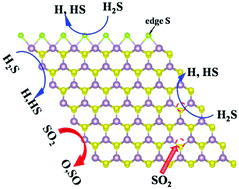
This study focuses on the modulation effects of S vacancy and Mo edges on the adsorption and dissociation behaviors of toxic gases (H2S and SO2) on MoS2 by first-principles calculations. Both molecules are found to chemisorb at the S vacancy (SV) and pristine Mo edge and physisorb at the Mo edge with a 50% sulfur coverage (Mo-50 edge). Among them, SO2 has larger adsorption energy than H2S on both S vacancy and pristine Mo edge, which is related to a more electronegative O than S atom and electronically rich for the pristine Mo edge. The defective states of MoS2 induced by SV can be removed by forming Mo–S, Mo–O and Mo–H bonds upon the adsorption of SO2 and the dissociation of H2S, which is applicable in designing MoS2 based nano-electronics devices in the future. The dissociations of H2S and SO2 on pristine Mo edges are found to be more favorable than those on S vacancies due to the catalytically active Mo4+ states at edge sites. H2S is found to dissociate on the Mo-50 edge more easily than SO2. The adsorptions and dissociations of toxic gas on MoS2 with SV or Mo edges suggest MoS2 is a potential candidate in detecting and removal of toxic gases.


 Please wait while we load your content...
Please wait while we load your content...