Polar zinc oxide surface in electrolyte solutions: an atomic view of reconstruction, hydration and surface states†
Abstract
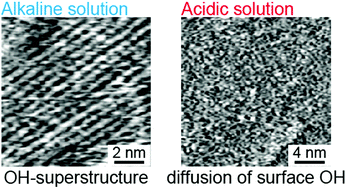
The stabilization mechanism of the Zn-terminated (Zn-) ZnO(0001) surface in electrolyte solutions has been investigated by using atomic-resolution liquid-environment atomic force microscopy (AFM) and an electrochemical method. The electrochemically measured pH dependence of the flat band potential of the Zn–ZnO(0001) surface indicated the adsorption of OH groups onto the (0001) surface in the wide pH range of 1–13. Atomic-scale AFM images of the Zn–ZnO(0001) surface showed a well-ordered hydroxide superstructure in an alkaline solution but a disordered structure in an acidic solution, which is probably attributed to the rapid diffusion of the adsorbed OH groups. Furthermore, the density of the O-terminated step edge on the Zn–ZnO(0001) surface in an acidic solution was higher than that in an alkaline solution. From these findings, we concluded that the excess positive charges of the Zn–ZnO(0001) surface are compensated by the adsorbed OH groups and the O-terminated step edges. In acidic solutions, a higher density of the O-terminated step edge is required for charge compensation. In addition, it was found that potential-dependent reversible surface reconstruction occurs in the local transition area with disordered step orientation by electrochemical AFM. We concluded that the reconstruction compensates the excess surface charges of the local transition area which are induced and varied by potential-dependent local surface states.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...